Atsushi Nambu, The 26th Recipient of the Tokizane Award in 2024
Movement control functions of the basal ganglia, pathophysiology of their disorders, and therapeutic applications
Atsushi Nambu
Division of Behavioral Development, National Institute for Physiological Sciences
Division of Behavioral Development, National Institute for Physiological Sciences
It is my great pleasure and honor to get an award named after Prof. Toshihiko Tokizane, who was a pioneer in the field of neurophysiology in Japan and the author of “Nou no hanashi (the tale of the brain)”, which I read in my youth.
When I was an undergraduate student in the late 1970’s, it was already known that the basal ganglia played important roles in controlling voluntary movements, as their dysfunctions caused movement disorders, such as Parkinson’s disease. However, their functions and dysfunctions were not well understood. Prof. Kazuo Sasaki, my mentor in the graduate university, suggested me to pursue research on the basal ganglia, because at that time, the basal ganglia were left uninvestigated, unlike the cerebral cortex and cerebellum. Since then, I started research in the field of the basal ganglia.
Following my research training in acute experiments under anesthesia, I utilized electrical stimulation in chronic awake experiments using behaving monkeys. When investigating neuronal activity of the output station of the basal ganglia, the internal segment of the globus pallidus (GPi), I first identified cortical inputs in GPi neurons by response to electrical stimulation in the cerebral cortex, and then recorded neuronal activity during motor tasks. In these recordings, I noticed that GPi neurons showed a triphasic response composed of early excitation, inhibition, and following late excitation in response to cortical stimulation. I assumed that the early excitation could be mediated by the subthalamic nucleus (STN).
Then, after two years of research in slice experiments in the basal ganglia at New York University, I started systematic examination of cortical inputs to the STN by using electrophysiological and anatomical methods with Dr. Masahiko Takada and others in monkeys. We found that the primary motor cortex and supplementary motor area directly projected to the STN in keeping with the somatotopic organization, and named this pathway as the “hyperdirect pathway”, which was distinct from trans-striatal pathways, namely the direct and indirect pathways. Moreover, pharmacological blockade of each pathway revealed that early excitation, inhibition, and late excitation in the GPi induced by cortical stimulation were mediated by the hyperdirect, direct, and indirect pathways, respectively.
We supposed that these pathways could work when executing voluntary movements as well, and proposed the "Dynamic activity model" of the basal ganglia functions: First, signals through the hyperdirect pathway reset on-going cortical activity; second, those through the direct pathway release necessary movements by disinhibiting thalamus; and finally, those through the indirect pathway stop movements.
On the other hand, malfunctions of the basal ganglia cause movement disorders, such as Parkinson’s disease and dystonia. We considered that their pathophysiology could be explained by changes in triphasic response patterns in the GPi. Until then, pathophysiology of movement disorders had been explained by firing rate or firing pattern changes of the basal ganglia, but firing rate changes were not obvious, and firing pattern changes did not properly explain their pathophysiology.
We recorded neuronal activity of parkinsonian monkeys as a starter. Treatment of monkeys with dopaminergic neurotoxin, MPTP, induced motor signs similar to those in human patients. We found that cortically induced inhibition in the GPi was reduced in parkinsonian monkeys (see Figure). This could be interpreted that in the normal state, cortically induced inhibition in the GPi releases movements by disinhibiting thalamus, while in the parkinsonian state, reduced inhibition in the GPi cannot release movements, resulting in akinesia/bradykinesia.
In contrast to Parkinson's disease, there are other movement disorders exhibiting involuntary movements, such as dystonia. We recorded neuronal activity from a mouse model of dystonia, which over-expresses human abnormal proteins, and we found that cortically induced inhibition was enlarged, and late excitation was reduced in the GPi (see Figure). This could be interpreted that enlarged inhibition releases excessive movements, and reduced late excitation is not enough to stop movements, resulting in involuntary movements.
Moreover, making a small lesion or applying continuous electrical stimulation in the basal ganglia ameliorate motor symptoms of advanced Parkinson’s disease. Likewise, pharmacological blockade of STN activity in parkinsonian monkeys recovered cortically induced inhibition in the GPi and improved akinesia/bradykinesia (see Figure). This could be interpreted that recovered inhibition in the GPi can release movements again.
Thus, the "Dynamic activity model" can explain not only the pathophysiology of movement disorders, but also the therapeutic mechanism of stereotactic neurosurgery. If we can find methods to normalize altered triphasic response patterns in movement disorders, that could lead to new therapeutic tools, and we will pursue the development of new tools in the future.
Lastly, I would like to send my deep appreciation to my mentors, colleagues, collaborators, and graduate students who have worked together with me during my research career.
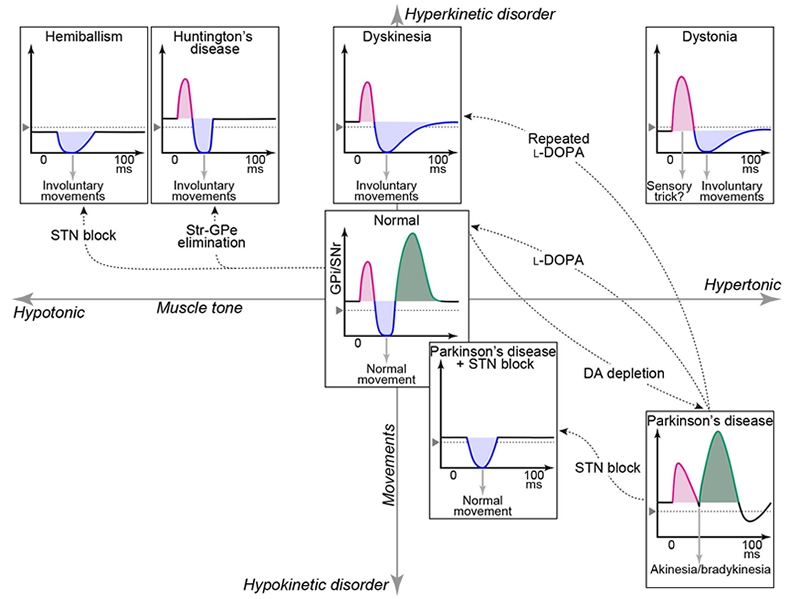
"Dynamic activity model" of movement disorders
Cortically induced response patterns in the output nuclei of the basal ganglia, the internal segment of the globus pallidus and substantia nigra pars reticulata (GPi/SNr), in various movement disorder models are plotted along hyperkinetic-hypokinetic (ordinate) and hypertonic-hypotonic (abscissa) axes. Alterations of cortically induced dynamic activity in the GPi/SNr could explain the pathophysiology of movement disorders in a unified manner. This model could also explain the therapeutic mechanism of stereotactic surgery targeting the subthalamic nucleus (STN) in Parkinson’s disease.
Related References
Nambu A et al. (1996) J Neurosci 16: 2671
Nambu A et al. (2000) J Neurophysiol 84:289
Nambu A et al (2002) Neurosci Res 2002;43:111
Chiken S et al. (2008) J Neurosci 28:13967
Nishibayashi H et al. (2011) Mov Disord 26:469
Sano H et al (2013) J Neurosci 33:7583
Chiken S et al. (2013) J Neurosci 33:2268
Chiken S et al (2015) Cereb Cortex 25:4885
Koketsu D et al. (2021) J Neurosci 41:5502
Chiken S et al (2021) Cereb Cortex 31:5363
Dwi Wahyu I et al. (2021) J Neurosci 41:2668
Darbin O et al. (2022) Sci Rep 12:6493
Hasegawa T et al. (2022) Nat Commun 13:2233.
Nambu A et al (2023) Mov Disord 38: 2145
Nambu A et al. (2000) J Neurophysiol 84:289
Nambu A et al (2002) Neurosci Res 2002;43:111
Chiken S et al. (2008) J Neurosci 28:13967
Nishibayashi H et al. (2011) Mov Disord 26:469
Sano H et al (2013) J Neurosci 33:7583
Chiken S et al. (2013) J Neurosci 33:2268
Chiken S et al (2015) Cereb Cortex 25:4885
Koketsu D et al. (2021) J Neurosci 41:5502
Chiken S et al (2021) Cereb Cortex 31:5363
Dwi Wahyu I et al. (2021) J Neurosci 41:2668
Darbin O et al. (2022) Sci Rep 12:6493
Hasegawa T et al. (2022) Nat Commun 13:2233.
Nambu A et al (2023) Mov Disord 38: 2145

Atsushi Nambu
Division of Behavioral Development, National Institute for Physiological Sciences
Division of Behavioral Development, National Institute for Physiological Sciences
Short CV
| 1982 | Graduate School, Faculty of Medicine, Kyoto University |
| 1985 | Instructor, Faculty of Medicine, Kyoto University |
| 1989 | Postdoctoral Fellow, New York University Medical Center |
| 1991 | Associate Professor, National Institute for Physiological Sciences, |
| 1995 | Staff Scientist, Director, Tokyo Metropolitan Institute for Neuroscience |
| 2002 | Professor, National Institute for Physiological Sciences, The Graduate University for Advanced Studies (SOKENDAI) |
| 2023 | Professor Emeritus, Researcher, National Institute for Physiological Sciences |





